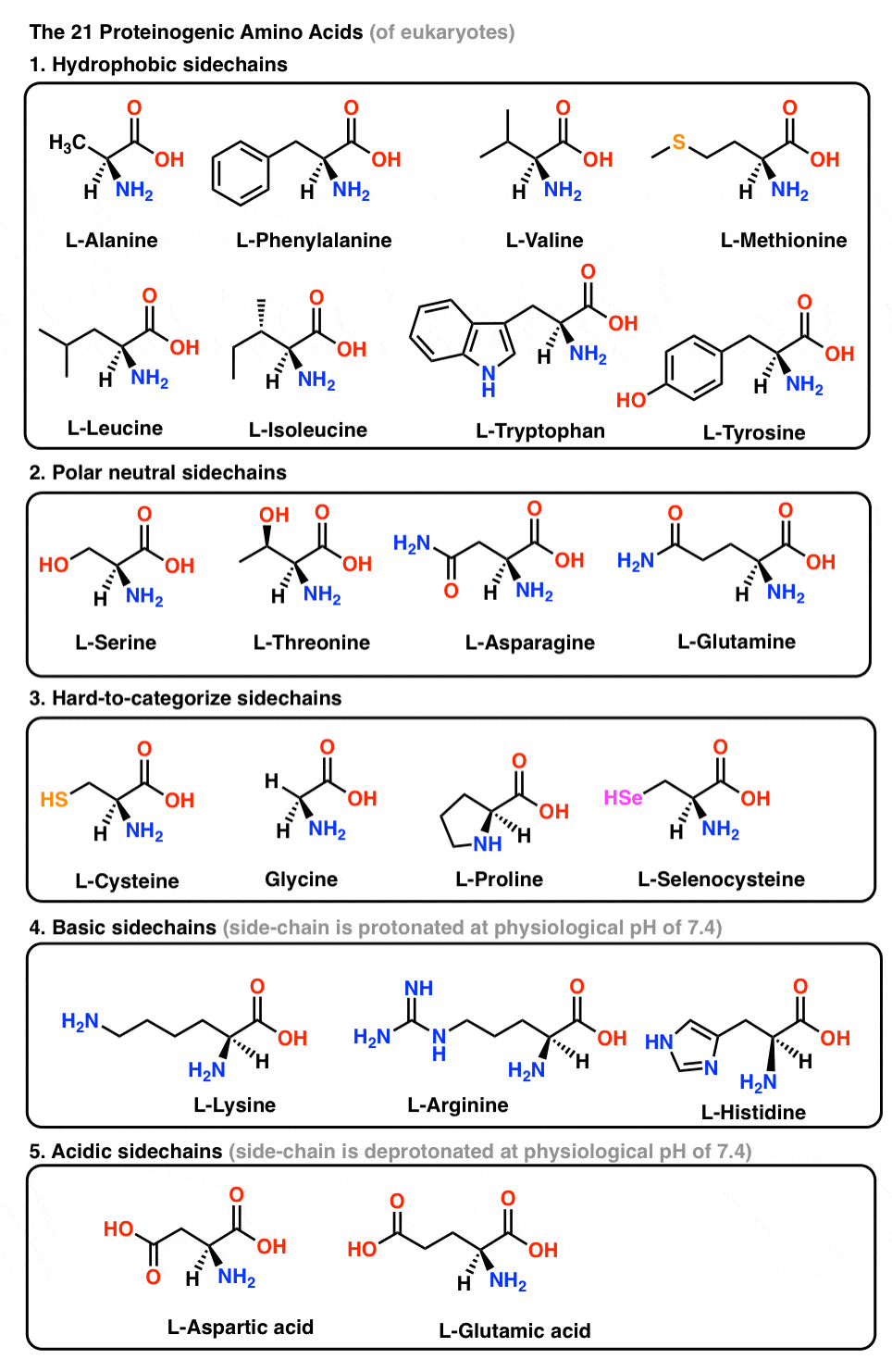
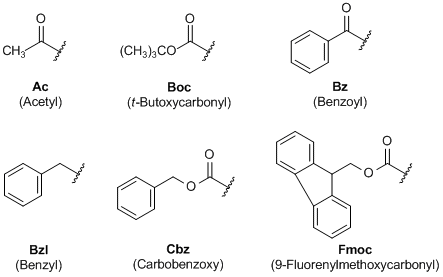
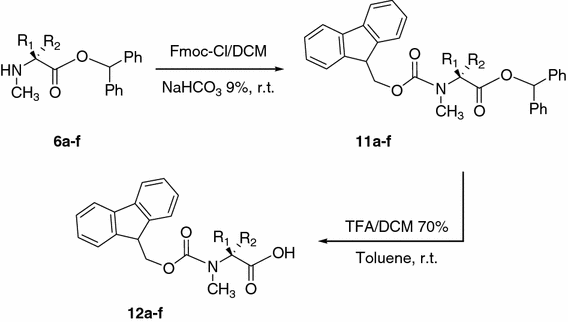
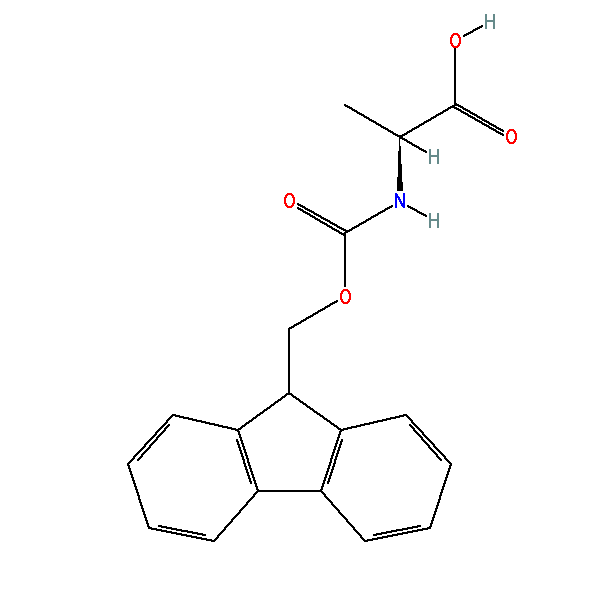
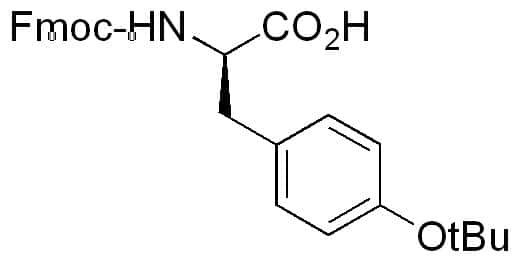
Large-Scale Syntheses of FMOC-Protected Non-Proteogenic Amino Acids: Useful Building Blocks for Combinatorial Libraries | Organic Process Research & Development

FMOC-Protected Amino Acids at best price in Kanchipuram by S.V.Chem Intermediates Private Limited | ID: 7215055933

Synthesis of Fmoc-protected 4-N,N,-dimethylaminophthalimidoalanine (1)... | Download Scientific Diagram
Fmoc-L-Glutamic acid-(OtBu) monohydrate, 5 g, CAS No. 204251-24-1 | Fluorenylmethylene / Fmoc | Amino acids, protected | Amino Acid Derivatives | Amino Acids and Amino Acid Derivatives | Organic & Bioorganic Chemicals | Chemicals | Carl Roth ...
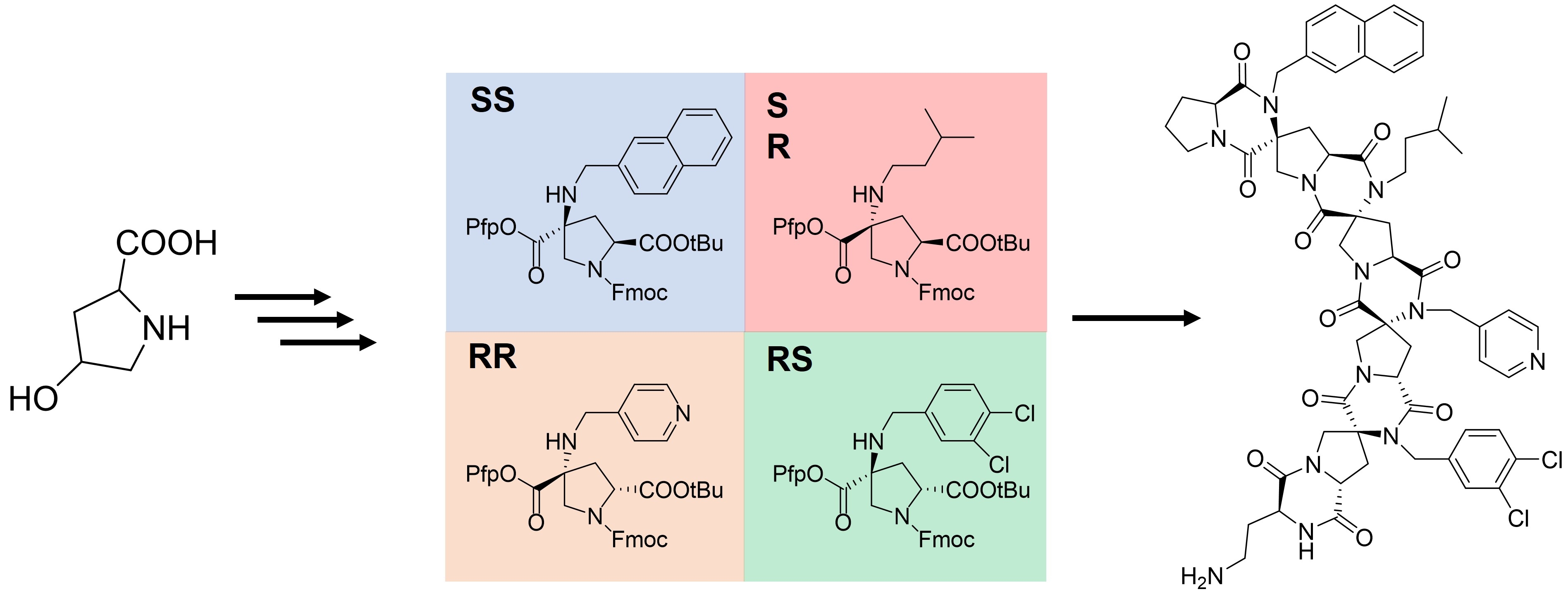
An upscaled synthesis of Fmoc-protected bis-amino acids for highly functionalized spiroligomers - American Chemical Society

Draw the mechanism for the reaction that removes a Fmoc group from an amino acid under these conditions. | Homework.Study.com
![PDF] Fmoc-2-mercaptobenzothiazole, for the introduction of the Fmoc moiety free of side-reactions. | Semantic Scholar PDF] Fmoc-2-mercaptobenzothiazole, for the introduction of the Fmoc moiety free of side-reactions. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/3232d408ed68af4d7d71eaef2557203a4a59a684/5-Figure2-1.png)
PDF] Fmoc-2-mercaptobenzothiazole, for the introduction of the Fmoc moiety free of side-reactions. | Semantic Scholar

Fmoc-protected amino acids as luminescent and circularly polarized luminescence materials based on charge transfer interaction - ScienceDirect
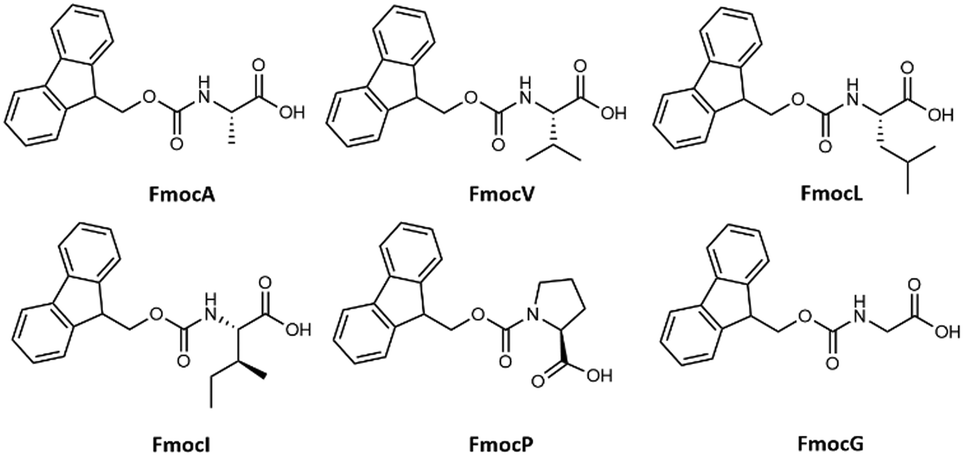
Solvent-controlled self-assembly of Fmoc protected aliphatic amino acids - Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/D2CP05938J

Molecules | Free Full-Text | Efficient Fmoc-Protected Amino Ester Hydrolysis Using Green Calcium(II) Iodide as a Protective Agent
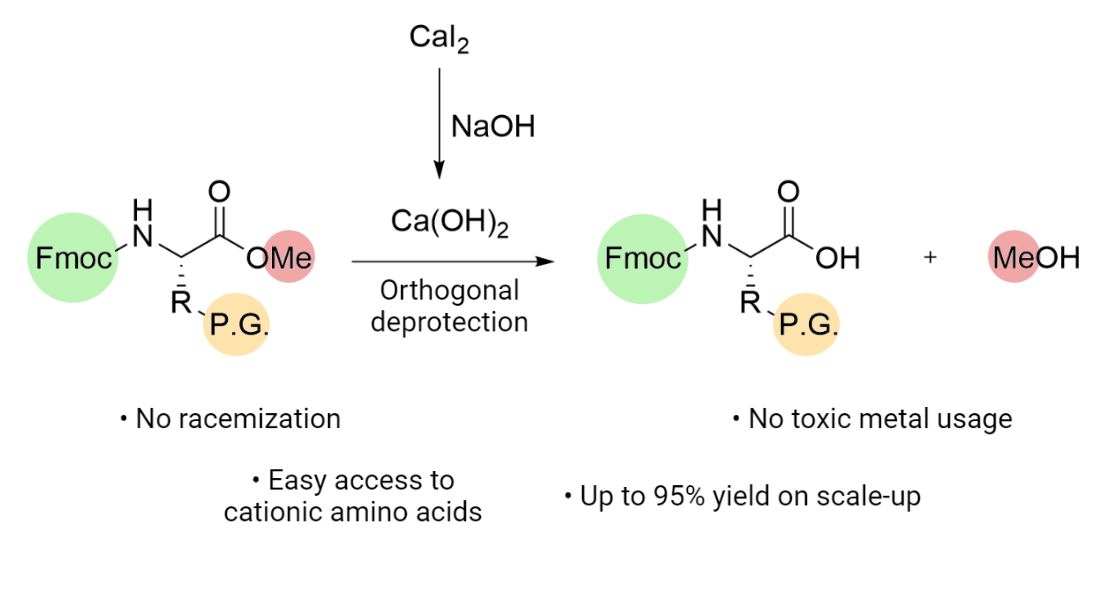
Molecules | Free Full-Text | Efficient Fmoc-Protected Amino Ester Hydrolysis Using Green Calcium(II) Iodide as a Protective Agent

An efficient and expeditious Fmoc protection of amines and amino acids in aqueous media - Green Chemistry (RSC Publishing)