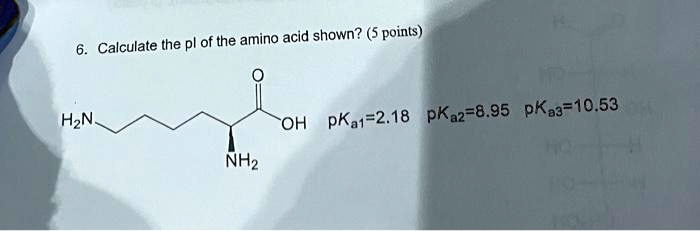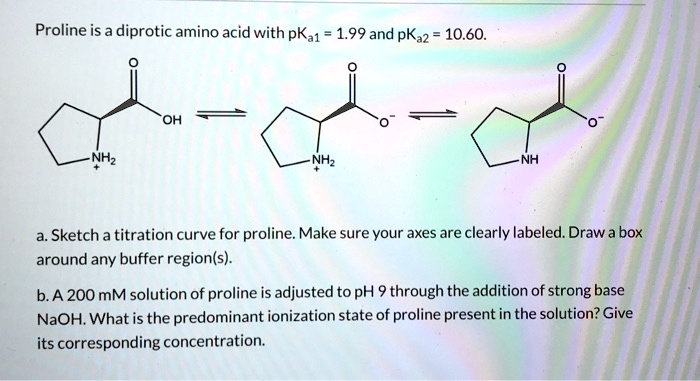
SOLVED: Proline is a diprotic amino acid with pKa1 1.99 and pKa2 10.60. OH NHz NHz Sketch a titration curve for proline. Make sure your axes are clearly labeled Draw a box

Titration of Amino Acids | pH, pKa1 and pKa2 | Amino Acids (Part 4). | By Medicosis Perfectionalis | Facebook

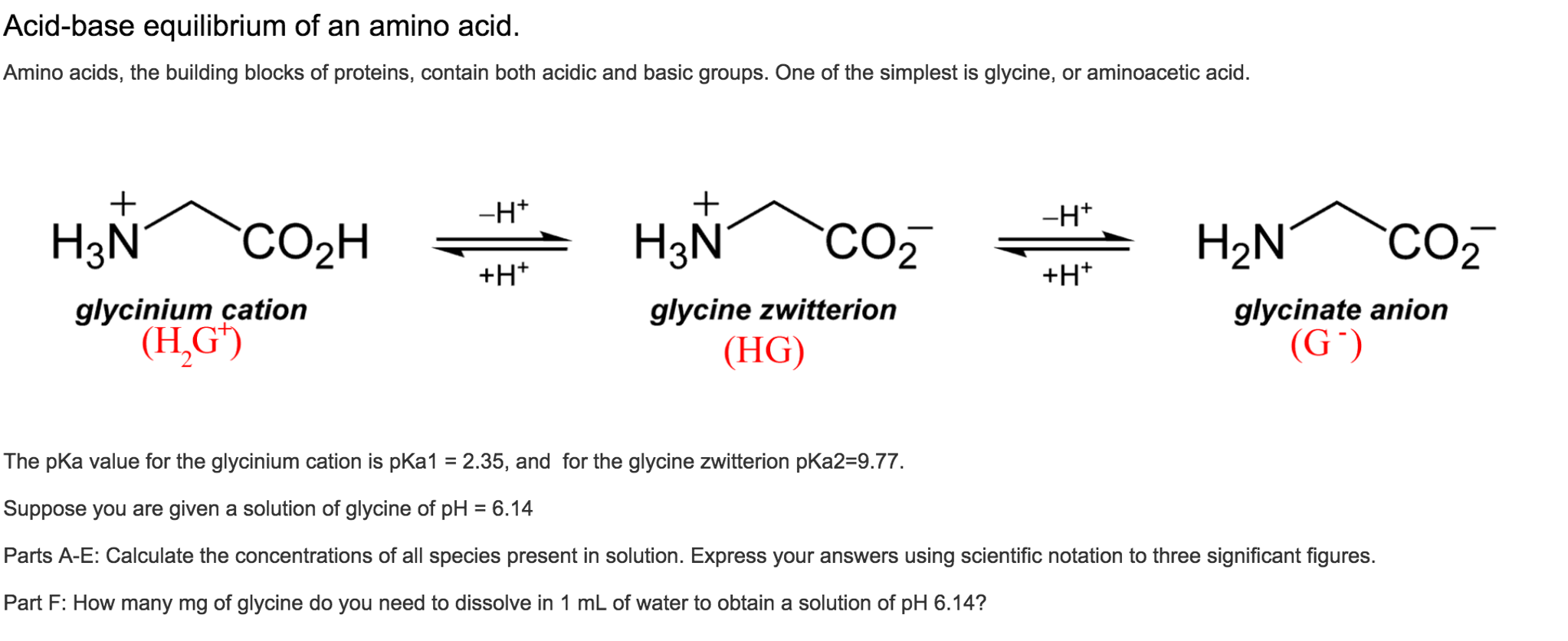
Consider the following ionic equilibriumGiven pKa1 =2.3 and pKa2 =9.7Then what is the isoelectric point of alanine?

H OH - 2 Charge: +1 Charge: 0 (When aa have a net charged of zero its called a Zwitterion ) Charge: -1 Low PHHigh PH Adding a base PH=1 PH=7 PH=12 Pka= ppt download

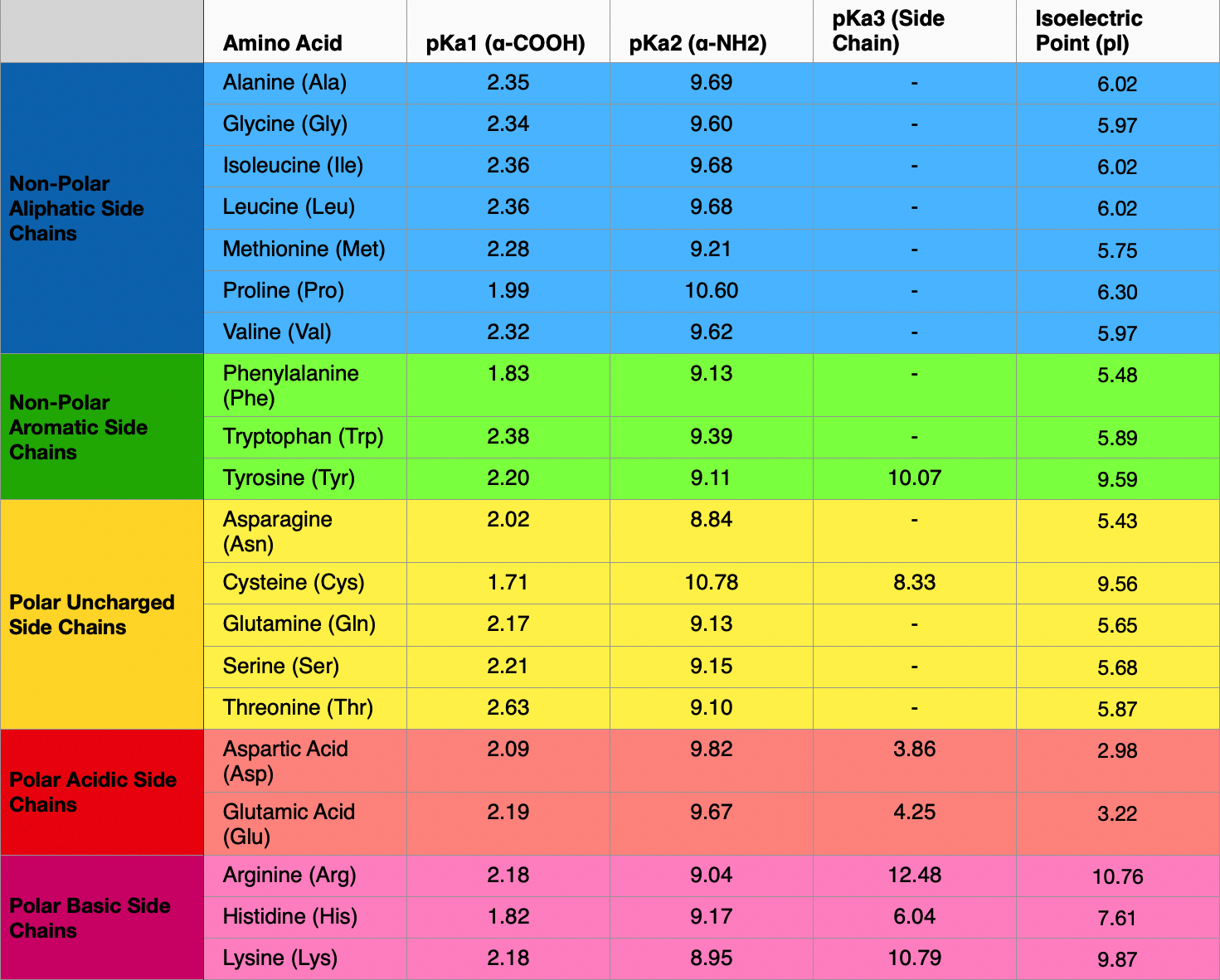
The amino acid methionine has pKa1 = 2.2 and pKa2 = 9.1. If this amino acid is represented by H2L+, what is the major species at pH 6? | Homework.Study.com
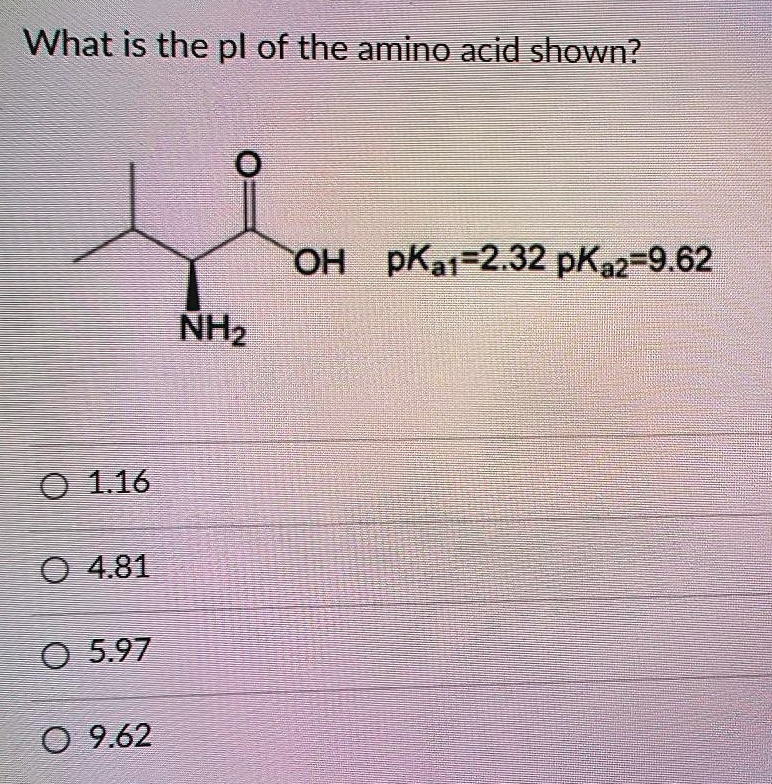
SOLVED: What is the pKa of the amino acid shown? OH pKa1 = 2.32, pKa2 = 9.62 NH2 1.16 4.81 5.97 9.62
How is an isoelectric point calculated in amino acids containing three amino or carboxyl group? - Quora